Turning Breakthroughs into Lifesaving Care—Faster
We don’t just give grants—we accelerate science, solve challenges, and connect experts who can bring breakthroughs to children years sooner.
At the Children’s Hospital of Philadelphia (CHOP), one of the world’s leading pediatric cancer centers, researchers discovered that an oral medicine called lorlatinib, originally developed for adults with lung cancer, showed promise against neuroblastoma tumors with changes in the ALK gene. Neuroblastoma is an aggressive childhood cancer that mostly affects children under the age of five. Some neuroblastomas have a mutation of the ALK gene which can make the tumor more difficult to treat, and these children face a worse prognosis.
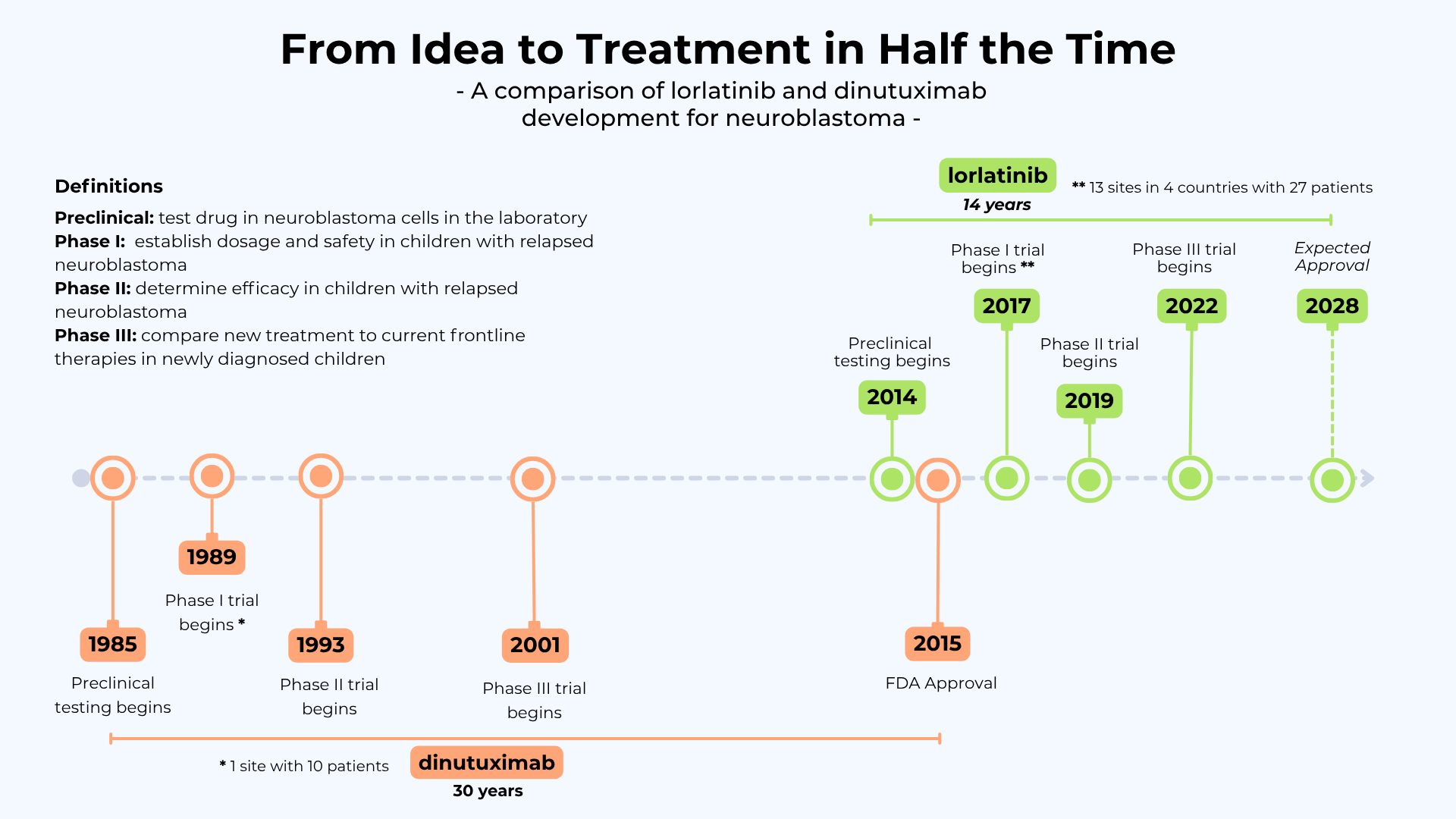
But moving a drug from adult use to children is not easy. Children’s bodies process medicine differently, and researchers must first prove the right dose is both safe and effective. Shockingly, the vast majority of drugs used to treat pediatric cancers are old chemotherapies developed decades ago for adult cancers and are not specifically approved for the unique cancers that occur in children. Only a handful of drugs have ever been developed and approved for childhood cancers, with the notable example of dinutuximab, an antibody approved for neuroblastoma in 2015 that took more than 30 years to test and gain FDA approval (shown in timeline below). This complex process is unacceptably lengthy and children with aggressive cancer simply don’t have that time. That’s where Solving Kids’ Cancer (SKC) stepped in.
From Idea to Trial in Record Time
In 2015 SKC attended a scientific meeting in Atlanta where researchers from CHOP first presented their early promising lab results of lorlatinib on neuroblastoma models and SKC immediately recognized the potential. SKC quickly worked with charity partners and donors to secure the funding needed to launch the very first pediatric trial in children with relapsed or treatment-resistant disease. Importantly, SKC also insisted that the trial include sites in the UK and EU to broaden access.
New Approaches to Neuroblastoma Therapy (NANT) consortium opened this Phase I trial in 2017 at 13 sites in 4 countries, including sites in London and Paris. This was accomplished remarkably fast, within two years of the initial lab results. For children, that kind of speed and access is critical.
Results of the NANT trial: The Phase 1 study showed that lorlatinib, an easy to administer oral pill, was safe with minimal side effects for children and young adults with relapsed or treatment-resistant neuroblastoma. Even more importantly, many children’s tumors shrank — and some disappeared entirely.
A Bold Challenge to Researchers: Transatlantic Frontline Trial
In 2019, in their continued push for advances in frontline therapies for children, SKC issued a bold call for proposals to grant $1.4 million for a transatlantic frontline therapy with the commitment of six charity partners. The award was announced Nov 2020, and the Phase III trial using lorlatinib opened May 2022. Once European enrollments begin, children diagnosed with ALK-driven neuroblastoma will be able to receive lorlatinib at hundreds of hospitals in more than 30 countries. This effort is expected to lead to an FDA and EMA approved drug for children upon diagnosis in the next few years.
Why It Matters
SKC recognized early on that lorlatinib was an effective agent, and by stepping in to fund, connect, and accelerate the research, shifted the field years sooner than it would have happened otherwise.
Today, thanks to donor support:
- A process that can take decades was cut in half.
- Newly diagnosed children are receiving lorlatinib throughout North America and will soon across Europe.
- Compared to toxic and debilitating chemotherapy, this once-a-day pill can be taken at home, with minimal to no side-effects.
- Because of SKC, families have new hope against one of the most devastating childhood cancers.
A Patient's Perspective
For families facing neuroblastoma, every day matters. Each scan, each treatment, each decision is accompanied by enormous trauma. Thanks to SKC donors, children and teens are now receiving lorlatinib—an option that once seemed out of reach. For parents, knowing that SKC collaborates with the world's top researchers to drive advances on behalf of their child offers something priceless: hope.
Click the photo below to hear firsthand from Kira, a teen from Scotland, who had suffered years of chemotherapy and numerous relapses. She has been taking lorlatinib for years and is now living her best life!